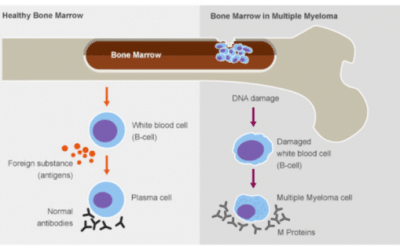
Burden of Multiple Myeloma Patient Care on Health Care in Curaçao Background Multiple myeloma (MM) is a hematological malignancy characterized by clonal proliferation of plasma cells in the bone marrow resulting in end-organ damage with morbidity and...
Oncology
Combined Modality Treatment
Outcome of Combined Modality Treatment Employing Modern Chemotherapy with Cobalt Based Radiotherapy in Curaçao Background Many cancers are currently treated with the combination of radiotherapy and chemotherapy as a radiosensitizer (combined modality treatment,...